Articles
See All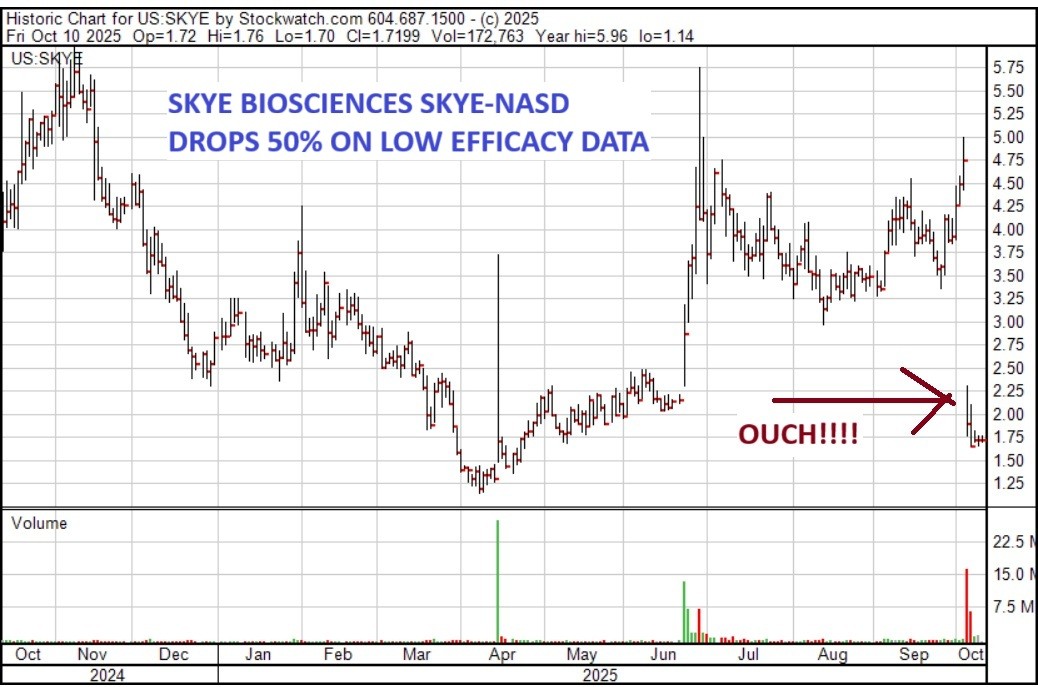
THE SKYE FELL AFTER MUTED INTERIM DATA
Skye Bioscience (NASDAQ: SKYE) released its Phase 2a results for its experimental obesity drug, nimacimab, and while the outcome for their drug candidate is mixed, the outcome for the stock was not–it lost 50% instantly. (That’s why this was such a small position).
However, for now, I have sold my position at $1.85. It rarely pays me to hold on to a losing position. I will continue to follow the story out of the portfolio and if I think the stock is worth re-purchasing, I’ll let you know.
While the drug did not meet its main goal as a stand-alone (monotherapy) weight loss treatment, analysts and the company believe there’s still real promise—especially when nimacimab is used together with GLP-1 drugs like Wegovy.
The data disappointed investors at first, sending shares sharply lower, but the science behind it is still an interesting story. Skye’s approach targets a completely different pathway from GLP-1 drugs—and if refined at higher doses or in combinations, nimacimab could still carve out a valuable place in the next generation of weight-loss treatments.
What Skye Bioscience Does
Skye Bioscience is a small biotech focused on a receptor called CB1—a key part of the body’s endocannabinoid system, which helps regulate appetite, fat storage, and metabolism. Earlier attempts to block this receptor (such as Sanofi’s Acomplia in the mid-2000s) were stopped because of psychiatric side effects like anxiety and depression.
Skye’s innovation is that nimacimab is a next-generation, “peripherally restricted” antibody—it blocks CB1 receptors in fat and liver tissue but does not enter the brain, where those psychiatric issues were triggered. This design is meant to keep the benefits of CB1 inhibition—better fat burning and metabolic control—without the mental health risks.
The Phase 2a Results: What Happened
The trial, called CBeyond, enrolled 136 adults with obesity or overweight, who received either nimacimab alone, placebo, or nimacimab combined with Wegovy (semaglutide). Each participant was treated for 26 weeks.
• Nimacimab monotherapy: Patients on nimacimab alone lost about 1.3% more body weight than placebo, a result that was not statistically significant (p=0.27). This was well below the company’s goal of 5–8% weight loss. Analysts from Piper Sandler and William Blair noted that the low efficacy was likely due to a too-low dose (200 mg weekly), as drug exposure in blood samples was much lower than predicted.
• Combination with Wegovy: When nimacimab was added to Wegovy, average weight loss reached 13.1% versus 10.0% for Wegovy alone, a difference that was statistically significant (p≈0.037). Importantly, this combination led to a better quality of weight loss, meaning patients lost more fat than muscle. In the Wegovy-only group, 28% of lost weight came from muscle; in the combination group, only 24% did.
Nimacimab was well tolerated, with no increase in psychiatric or gastrointestinal side effects compared to placebo. There was only one case of mild insomnia in both the nimacimab and placebo arms, and overall discontinuation due to side effects was low.
Safety First, Then Efficacy
Despite the disappointment on efficacy, the safety data is a major win for Skye. Historically, CB1 blockers failed because they caused anxiety, depression, and sleep problems. Nimacimab showed none of these issues, even at long-term exposure. Analysts see this as a “green light” to test higher doses (600–1,000 mg weekly) in the next trial.
The company has already started an open-label extension of the current study, where participants will now receive a 300 mg weekly dose to determine whether higher blood levels lead to better results. These data are expected in the first half of 2026, followed by a larger Phase 2b trial at higher doses later that year.
Why This Approach Is Different From GLP-1 Drugs
GLP-1 drugs like Wegovy and Mounjaro have been game changers for obesity, but they mainly work by slowing digestion and reducing appetite, leading to lower calorie intake. However, they don’t directly change how the body burns fat—and they can also cause side effects like nausea, vomiting, and muscle loss.
Nimacimab works through a totally different mechanism:
• It blocks CB1 receptors in fat tissue and the liver, which promotes fat breakdown and helps prevent the liver from storing more fat.
• Because it doesn’t enter the brain, it avoids psychiatric side effects seen with older CB1 drugs.
• When combined with a GLP-1 drug, nimacimab may amplify total fat loss while preserving muscle mass.
Analysts describe it as potentially becoming a “fat burner complement” to appetite-suppressing GLP-1s—a pairing that could help patients achieve faster and healthier weight loss with fewer side effects.
What Analysts Are Saying
Most analysts lowered their price targets on the stock following the release, but remain moderately optimistic about the drug’s potential if dosing is fixed:
• Piper Sandler cut its target from $20 to $13, noting that the 200 mg dose was clearly suboptimal but that the drug’s clean safety and synergy with Wegovy are encouraging.
• William Blair reiterated its Outperform rating, saying the “risk/reward profile is skewed to the upside” now that the stock has dropped 50%, and expressing confidence that higher doses could reveal meaningful efficacy.
• JMP Securities also maintained a positive stance, saying nimacimab “needs more work” but still holds potential, particularly in combination with incretin drugs.
• LifeSci Advisors’ media roundup noted that while headlines focused on the miss, outlets like BioWorld and MedCity News highlighted Skye’s intent to pursue higher doses and new combination trials.
Looking Ahead
Skye plans to present the full dataset at Obesity Week in November 2025, which should provide more details about patient subgroups and exposure levels. The 300 mg dose study will continue through early 2026, followed by a Phase 2b dose-ranging trial using 600–1,000 mg weekly.
Though the last financials showed roughly $49 million in cash, it’s likely they need to raise more $$ to study the Wegovy relationship in more detail to get FDA clearance. That puts a cap on the stock.
Bottom Line: Not a Breakthrough Yet, but Still Interesting
Skye’s Phase 2a results weren’t the home run investors hoped for, but the long term story isn’t over. Nimacimab has proven it’s safe, tolerable, and works well in combination with Wegovy—that’s a good foundation for a small biotech in this field. But investors can likely wait to see what the next round of data looks like without worrying about any big speculative premium building in the stock.
If higher doses confirm a dose-response relationship next year, Skye could reposition nimacimab as the first safe CB1-targeted adjunct therapy for use alongside GLP-1s—potentially improving weight loss, reducing muscle loss, and lowering side effects.
For retail investors, this means Skye remains a speculative but intriguing play in the weight-loss space. The science is sound, the safety profile is strong, and the next trials could determine whether nimacimab can move from “interesting idea” to real complementary therapy in a market worth tens of billions.
